TopPoint supplies disposable nurse caps for hospital hygiene and infection control under ISO 13485-certified quality systems in cleanroom environments.
Precision Medical Devices for Global Healthcare
TOP-POINT supplies a full spectrum of high-quality, disposable medical products,
manufactured under strict ISO 13485 standards for safety and reliability.
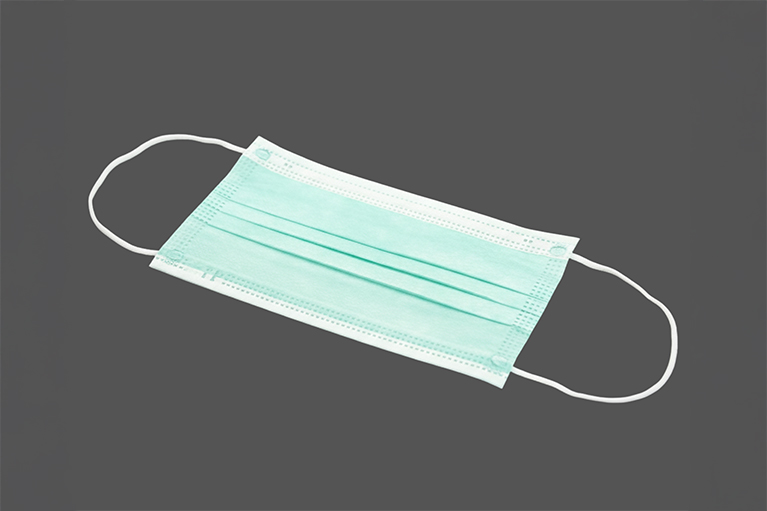
TopPoint manufactures sterile surgical masks for medical and hospital use, providing reliable filtration and produced under ISO 13485-certified quality systems.
TopPoint manufactures sterile suction catheters for airway care and suction procedures under ISO 13485-certified quality systems to ensure safe performance.
TopPoint provides sterile feeding tubes for enteral nutrition and patient care, produced under ISO 13485-certified quality systems for reliable gastrointestinal safety.
TopPoint manufactures medical stomach tubes for gastric drainage and suction, providing safe, sterile performance under ISO 13485-certified quality systems.
TopPoint manufactures sterile transfer spikes for infusion and transfusion use, ensuring safe fluid connections under ISO 13485-certified quality systems.
TopPoint provides 3-way stopcocks for IV and infusion sets, allowing precise fluid flow control and produced under ISO 13485-certified quality systems.
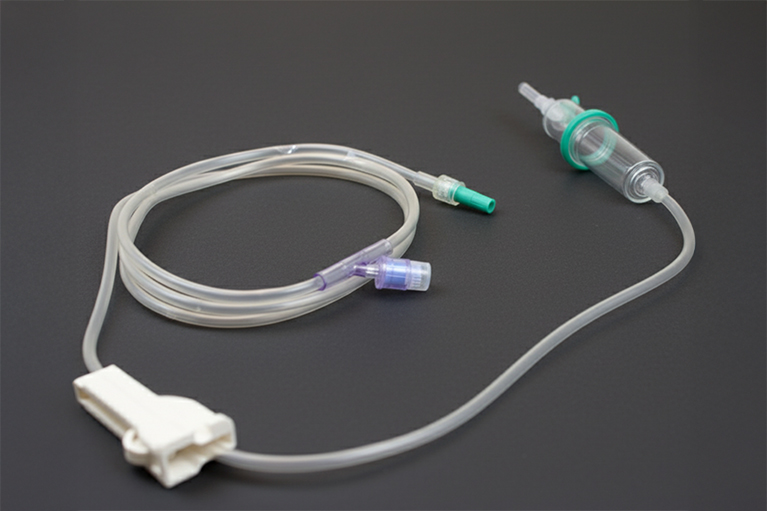
TopPoint manufactures IV extension lines for safe, flexible fluid transfer under ISO 13485-certified quality systems for medical infusion and transfusion procedures.
TopPoint provides safety infusion sets with needle protection and anti-backflow systems under ISO 13485-certified quality systems for safe IV fluid delivery.
Full-Cycle Medical Device Manufacturing Solutions
TOP-POINT supplies a full spectrum of high-quality, disposable medical products,
manufactured under strict ISO 13485 standards for safety and reliability.
High-speed automation for ISO-certified assembly.
Cost-effective, customized solutions for global partners.
Precision grinding technology for high-quality cannulas
End-to-end solutions: from feasibility to installation.
Follow our global footprint and commitment to innovation. This section features press releases,
exhibition participation, and key achievements that solidify our market leadership.
YOUNG CORPORATION: TOP-POINT will proudly participate in the 17th K-BEAUTY EXPO KOREA 2025, held at KINTEX from September 11th to 13th. This exhibition is a crucial platform to demonstrate our specialized automation machinery, such as high-precision filling systems, essential for the cosmetic manufacturing industry. We invite you to Visit us at HALL 7, Booth No. K14 to explore how our advanced technology supports the efficiency and quality of K-Beauty Technology. Our commitment extends beyond medical devices to innovative solutions for the entire beauty supply chain.
Young Corporation has been officially reappointed as a Designated Trading Company by the Ministry of Trade, Industry and Energy (MOTIE) of Korea. The 2025 Designation Ceremony, co-hosted by KOTRA and the Korea International Trade Association, was held on July 8, 2025, at COEX in Seoul.
Young Corporation successfully participated in Seoul Food 2025, held in Ilsan, Korea, and concluded the exhibition with great success. As one of the largest and most influential food industry exhibitions in the region, the event served as a key platform for introducing the latest innovations and technologies in food manufacturing.
YOUNG CORPORATION: TOP-POINT will be participating in SEOUL FOOD 2025, the International Food Industry Exhibition at KINTEX, Seoul, from June 10th to 13th. We invite you to Visit us at HALL 8, Booth No. 8I401 to explore our cutting-edge automation solutions, including high-precision filling and packaging machinery, specifically designed for the Food Technology and processing sector. This participation highlights our capability to apply our expertise, initially developed in medical manufacturing, to advance the global food industry's efficiency and quality.
Young Corporation successfully wrapped up its second participation in KIMES 2025, Korea’s leading international medical and hospital equipment exhibition held at COEX, Seoul. As one of the key players in the medical device field, Young Corporation once again drew attention with its innovative product lineup and customized solutions.
YOUNG CORPORATION: TOP-POINT is excited to announce its return to the 40th Korea International Medical & Hospital Equipment Show, KIMES 2025, held at COEX, Seoul. As a trusted leader in medical device manufacturing and automation, we invite you to Visit us at 1F, Hall B Lobby, Booth No. BL113. We will be showcasing our newest Innovative Medical Solutions, ranging from high-precision cannulas to advanced syringe manufacturing automation. Our sustained participation reaffirms our commitment to driving excellence and innovation in the global healthcare industry.
Young Corporation participated in MD&M WEST 2025, held in Anaheim, USA, marking its debut at this prestigious event. MD&M WEST is one of the world’s leading exhibitions for medical device manufacturing and design, offering a comprehensive view of the latest technologies and trends in the global medical device industry.
Young Corporation successfully participated in the 2024 World Food Tech Exhibition held in Korea. This significant event brought together food technology experts and companies from around the globe to showcase the latest technologies and products, and Young Corporation garnered substantial attention with its innovative machinery.
YOUNG CORPORATION: TOP-POINT proudly participated in the World FoodTech Expo 2024 at COEX, Seoul. This exhibition served as a critical platform to showcase our versatile automation machinery and solutions specifically tailored for the Food Technology and processing industry. We invite you to Visit us at HALL C, Booth No. C4301 to explore how our high-precision equipment, derived from our expertise in medical manufacturing, drives efficiency and innovation in the global food industry. Our commitment is to providing top-tier automated solutions across various sectors.
Find quick answers to common questions about our Turnkey Solutions, OEM services, ISO 13485 compliance, and product quality.
If you don't see your question, please Contact Our Experts.
What is included in your Medical Device Turnkey Factory Setup?
Our turnkey solution is comprehensive, covering everything from initial factory design and layout to the supply and installation of full automation machinery, cleanroom validation, and technical training for operations.
Are your manufacturing processes compliant with international quality standards?
Yes, all our manufacturing and quality management processes are certified under ISO 13485:2016. This guarantees the highest standards of safety and efficacy for all our medical devices.
Do you provide OEM and Contract Manufacturing services?
Absolutely. We offer full OEM and contract manufacturing services for various disposable medical devices, utilizing our ISO 13485-certified production facilities to meet your specific design and volume requirements.
What types of medical devices do you specialize in manufacturing?
Our core expertise lies in the production of high-precision disposable items, including syringes, hypodermic needles, cannulas, and various IV administration and transfusion sets.
Can you support factory set up different countries and customer's requirements?
Yes. We have experience working in various countries across Asia, the Middle East, Africa, the United States, and other regions. We provide flexible, fully customized turnkey plant solutions tailored to the local conditions and specific requirements of customers in various countries.